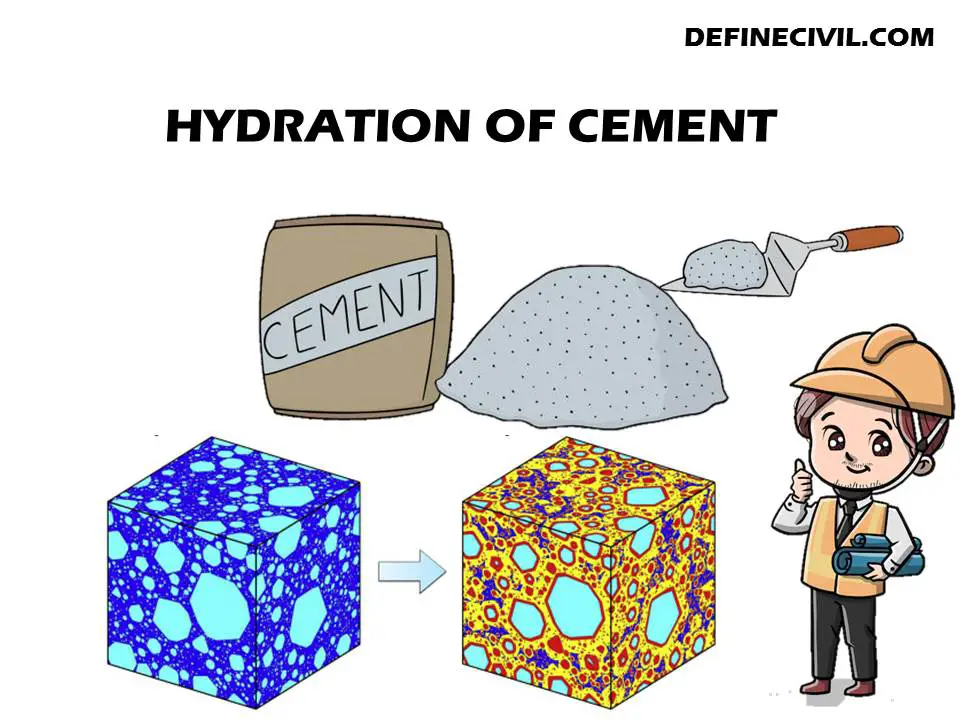
What happens when you mix cement with water or if it accidently gets some moisture? It becomes a paste for some time and then becomes hard like a rock-mass. This all happens because of a chemical reaction between cement chemicals and water – called hydration reaction.
The chemicals of cement (also called bogue’s compounds) are responsible for the hydration and hardening of cement mass. It’s an exothermic reaction, meaning it liberates heat energy that’s commonly referred to as heat of hydration.
So, in our today’s blog we’ll discuss in detail about what hydration of cement in civil engineering and what heat of hydration is and what is its importance in terms of durability of concrete.
Table of Contents
- What is hydration of cement?
- Hydration – as a process
- Water requirement for complete hydration of cement
- Properties of cement responsible for hydration
- Structure of cement
- Importance of Hydration of Cement:
- Hydration in Cement:
- Mechanism of Hydration of Cement:
- Cement chemical reaction formula
- Test for Cement to Check Quality:
What is hydration of cement?
Cement is the most commonly used construction material in the world. It is a mixture of sand, gravel, and limestone.
The hydration of cement is a chemical reaction between cement compounds (bogues compounds) to form chemical bonds with water molecules and forms hydration or hydration products.
So, hydration of cement is chemical reaction of cement with water molecules in an exothermic process.
The heat of hydration of cement is the amount of energy required to hydrate a mass unit of cement (1 kg) in water at 37°C.
This quantity is defined as the product of the enthalpy change and time, or ΔH value. The enthalpy change depends on temperature, pressure and type of cement used.
A higher enthalpic value implies that it takes more energy to make concrete from this type of material than from another one. However, a hydrated cement is one that can be used to make concrete.
Also Read: Initial & Final Setting time of cement
Hydration – as a process
It has been mixed with water and then placed in a kiln for the next step of making concrete. This process is called hydration, which means to become wet or damp.
The hydrated cement becomes moist as it absorbs moisture from the air around it during the drying process after being made into concrete. Hydration occurs when water molecules enter into the structure of an alkali-silica-carbonate compound (ASCC).
Water molecules are attracted to this type of material because they have a positive charge while carbon dioxide molecules have a negative charge; therefore, they attract each other.
Also Read: Difference between Cement and Concrete or Mortar
Water requirement for complete hydration of cement
For a normal Portland cement, about 23% of water by weight of cement is required for chemical reaction. For filling the gel pores, the cement needs minimum of 15% of water. So, in total you need to adjust the water-cement ratio such that the water percentage is 38% by weight of cement. This is the amount of water required for complete hydration and filling pores.
So, in terms of water-to-cement ratio, the minimum value is 0.36 w/c for the 100% hydration of cement. However, at this value the workability of the mass might not be good enough for site condition. So, it is recommended to keep a minimum value of w/c ratio of 0.42 to avoid water loss due to bleeding and evaporation. Anyhow, this threshold value also depends on the temperature of ingredients and the environment.
Now we can solve:
What is the water required for complete hydration of 150 kg cement?
As you know that for complete hydration of cement you require 38% water by wt of cement. So, 38*150/100 = 57 kg.
Also Read: Types of Cement – Classification based on standards
Properties of cement responsible for hydration
Cement is a hydrous silicate mineral, in which chemical formula is CaSiO3. It has a high melting point of approximately 1,400°F (760°C) and is usually gray or white.
Cement’s properties are determined by its chemical composition and physical condition; it also depends on the type of cement used.
Structure of cement
The structure of cement includes calcium silicates interspersed with variable amounts of oxygen and silicon dioxide. Hydration reactions between water molecules and SiO2 produce hydroxyl ions that react with CaSiO3 to form an intermediate complex called amorphous calcium silicate hydrates.
Hydration reduces the amount of cement required for making concrete by replacing some of it with water. This process also increases the workability of concrete so that it can be formed into more complex shapes than those that are possible with ordinary Portland cement (ordinary or standard Portland cement).
Importance of Hydration of Cement:
Hydration of cement is very important because it helps in the adhesion of concrete to the substrates.
- If hydration is not done properly, then the strength or durability of a concrete will be affected.
- It also increases the flexural and tensile strength of concrete and enhances its resistance to cracking by increasing its compressive strength (Strength).
- It also acts as an adhesive for other materials like steel rebar and reinforcing steel which are used in construction projects.
- When water is present in fresh cement, it reacts with calcium ions present in it and forms calcium hydroxide Ca(OH)2 which has antibacterial properties. The hydration of cement is a process that converts the calcium hydroxide Ca(OH)2 in the cement to calcium carbonate CaCO3. This occurs during mixing and as it sets.
The rate at which this happens depends on how much water was added, how long it was mixed, etc., depending on the type of cement being used.
Also Read: Harsh mix of concrete – Harshness in Concrete is due to
The rate of hydration is accelerated by temperatures above about 30°C (86°F). It also slows down when temperatures are below 0°C (32°F). The slower rates can be caused by an excessive amount of air or moisture in the mix.
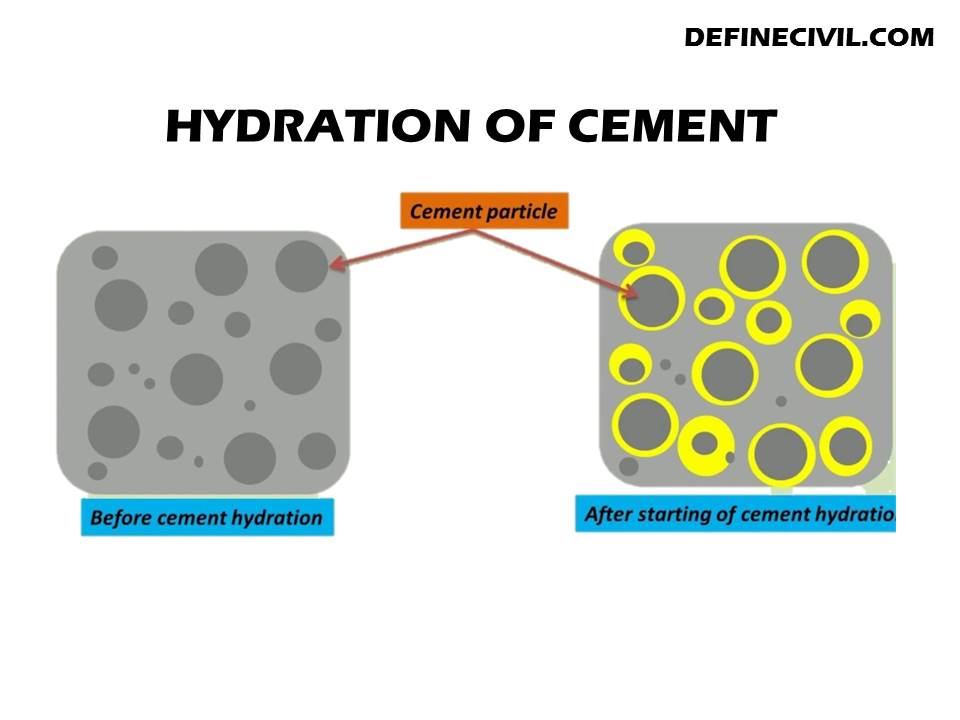
Hydration in Cement:
The cement is made up of fine particles. These are used to form concrete. The main components include silica fume, quartz, alumina, and sodium carbonate. Also, there are many other ingredients that make up the cement powder including calcium oxide, magnesium oxide, and aluminum hydroxide. There are different types of hydrations in cement which include
Rapid hydration of concrete is a process conducted in the laboratory that mimics what happens during construction. In this process, concrete is mixed with water and then allowed to harden in an oven at room temperature.
Also Read: What is Bogues Compounds in Cement
The mixture is then poured into molds or tubes and left to set for several days before being removed from the molds or tubes. The time required for rapid hydration depends on the size of the sample, but it can take anywhere from 7-10 days.
Rapid hydration does not produce a structure as strong as one produced through normal drying methods, but it does provide very fast results when compared to other types of drying.
This type of hydration is done in a lab and takes place within 24 hours of mixing. It includes a formula that has been tested by scientists for optimum performance with minimum water loss during the hydration process and also high strength after curing.
Mechanism of Hydration of Cement:
The cement is made up of calcium carbonate (CaCO3), which is the most abundant mineral in the Earth’s crust. When it reacts with water, a chemical reaction takes place known as hydration to form calcium hydroxide Ca(OH)2.
This process occurs due to the presence of water molecules in the cement particles and the ionic bonds between them.
Water molecules are attracted to each other by their hydrogen bonds and thus, they can be kept together even when they are mixed with different types of ions such as sodium ions present in concrete.
It is the process by which cement particles are transformed from a solid to a liquid substance. The mechanism involves chemical reactions, mainly hydrolysis and acid-base reactions that take place in the presence of water.
The rate at which this reaction occurs depends on several factors such as pH, temperature and concentration of reactants. If not controlled properly, it can lead to excessive loss of cement or premature setting up if too much water is added.
Also Read: Physical Properties of Cement to surprise you
Cement chemical reaction formula
The reaction that takes place during the hydration of cement is (CaO + 3MgO) → Ca(OH)2 + 4H2O.
This reaction happens at temperatures higher than 100oC, but if this temperature rises above 300oC, then this reaction stops.
Cement hydrates are not only responsible for making concrete strong and durable, but also control how long it takes for concrete to set up once poured.
Test for Cement to Check Quality:
There are different types of tests that can be performed in a laboratory to check the quality of cement. Some of them are as follows:
- Strength Test
- Hydration of Cement
- Soundness of Cement
- Specific gravity of cement
- Fineness of cement
- Consistency test of cement
Further Read: Difference between Segregation and Bleeding in Concrete